 While great strides have been achieved in cancer treatment, scientists are looking for the next generation of therapeutics to stop this second leading cause of death nationwide. A new approach has been developed through a collaborative effort linking chemists at NYU and pharmacologists at USC.
While great strides have been achieved in cancer treatment, scientists are looking for the next generation of therapeutics to stop this second leading cause of death nationwide. A new approach has been developed through a collaborative effort linking chemists at NYU and pharmacologists at USC.
In a study appearing the week of September 9 in the Proceedings of the National Academy of Sciences, the research groups of Bogdan Olenyuk of USC School of Pharmacy and Paramjit Arora of NYU College of Arts and Sciences have developed a synthetic molecule, “protein domain mimetic”, that targets the interaction between two proteins at the point where intracellular signaling converges resulting in an up-regulation of genes that promote tumor progression. This approach presents a new frontier in cancer research, and is distinct from the typical search for small molecules that target cancer cells.
“There is an urgent need to accelerate the discovery of mechanism-based anticancer therapeutics”, said Swati Kushal, a USC School of Pharmacy postdoctoral scholar who is a lead co-author on the paper. “Traditionally, transcription factors and their complexes have been extremely challenging targets for drug design, mostly because it has been inherently difficult to find synthetic molecules that bind to their shallow surfaces with high affinity and specificity. “
For the most part, efforts to design compounds that can inhibit human transcription factors have been largely unsuccessful. But this study reports a successful approach spanning from concept to in vivo studies.
“When we embarked on this study several years ago, we did not realize how incredibly complex this research would be,” noted Bogdan Olenyuk, co-corresponding author of the PNAS paper and assistant professor at USC Mann. “Our collaborative efforts led us to design a stabilized protein fold that must bind to its target transcription factor complex with high affinity, regulating the activity of a traditionally “untreatable” protein embedded in a highly complex cellular signaling network, correlating the in vitro and in vivo data and, lastly, analyzing the overall effects the compound produces.”
Of particular note, the synthetic molecule that the paper describes – HBS 1 – is based on a chemically stabilized protein that is mimicking the specific molecule on which it has been modeled and shows outstanding potential for tumor suppression. This compound was specifically designed to interrupt the type of molecular conversation within the cell -called cell signaling – that promotes growth of cancer cells. Creation of HBS 1 required a method for locking correct helical shapes in synthetic strings of amino acids – a method previously developed at NYU.
The studies conducted at NYU and USC proved that the new approach disrupted the cancer cell “conversation,” reached the correct target in the cell, resulting in a rapid blockade of tumor growth. Furthermore, the compounds did not show any signs of toxicity or negative impact in the test host.
“Despite the fact that targeted protein complex turned out to be very uncooperative and capricious in our biophysical experiments, the synthetic molecule HBS 1, much of our delight, behaved well and produced a rapid and sustained disruption of hypoxia-inducible signaling network in cells, which translated into a robust suppression of the rate of tumor growth in our mouse models” said Olenyuk. “This project would not be possible without the interdisciplinary collaboration between USC and NYU.”
While the in vivo experiments were conducted using renal cancer cells, the principles of this design are applicable to many human conditions, including other cancers, cardiovascular diseases and diabetic complications. The general concept of the study, the interruption of the connection between genes as they conspire to promote cancer growth, is general and applicable to the protein cell to protein cell “conversations” implicated in a host of human diseases.
Next, the USC and NYU teams will initiate the translational aspects of the project. The compounds will tested in advanced tumor models with the aim to ultimately take the compound into clinical trials. In addition to the lead authors on the paper, contributors include Ramin Dubey and Hanah Mesallati from USC and Brooke Bullock Lao, Laura K. Henchey and Nathaniel J. Traaseth from NYU.
Funding for this research came from National Science Foundation CAREER Award to Bogdan Olenyuk and a National Institutes of Health R01 grant to Paramjit Arora.
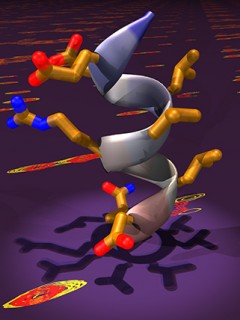
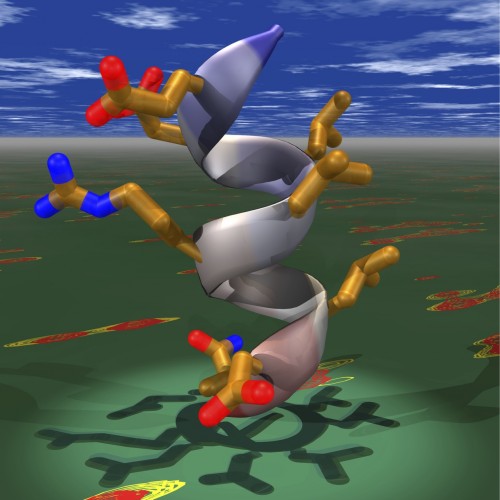 Image caption: Pictured is a ray-traced model of the αB helix of the C-terminal transactivation domain of hypoxia-inducible factor 1α (HIF -1α) juxtaposed with the spectrum of its complex with the coactivator protein p300, generated from heteronuclear single quantum correlation (HSQC) NMR experiment. Recruitment of the coactivators by HIF-1α is key step in transcription of hypoxia-inducible genes, many of which are implicated in cancer progression and in the development of macular diseases. Kushal et al. found that stabilized mimic of the αB helix can disrupt this transcription factor-coactivator interaction, resulting in tumor growth arrest. The results from this study could be used to design new tools for cell biology, genetics and transcription-based therapeutics. Image courtesy of Bogdan Olenyuk (University of Southern California).
Image caption: Pictured is a ray-traced model of the αB helix of the C-terminal transactivation domain of hypoxia-inducible factor 1α (HIF -1α) juxtaposed with the spectrum of its complex with the coactivator protein p300, generated from heteronuclear single quantum correlation (HSQC) NMR experiment. Recruitment of the coactivators by HIF-1α is key step in transcription of hypoxia-inducible genes, many of which are implicated in cancer progression and in the development of macular diseases. Kushal et al. found that stabilized mimic of the αB helix can disrupt this transcription factor-coactivator interaction, resulting in tumor growth arrest. The results from this study could be used to design new tools for cell biology, genetics and transcription-based therapeutics. Image courtesy of Bogdan Olenyuk (University of Southern California).

